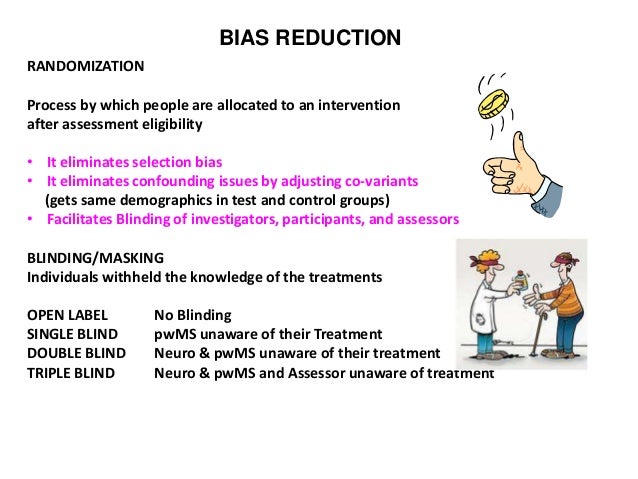
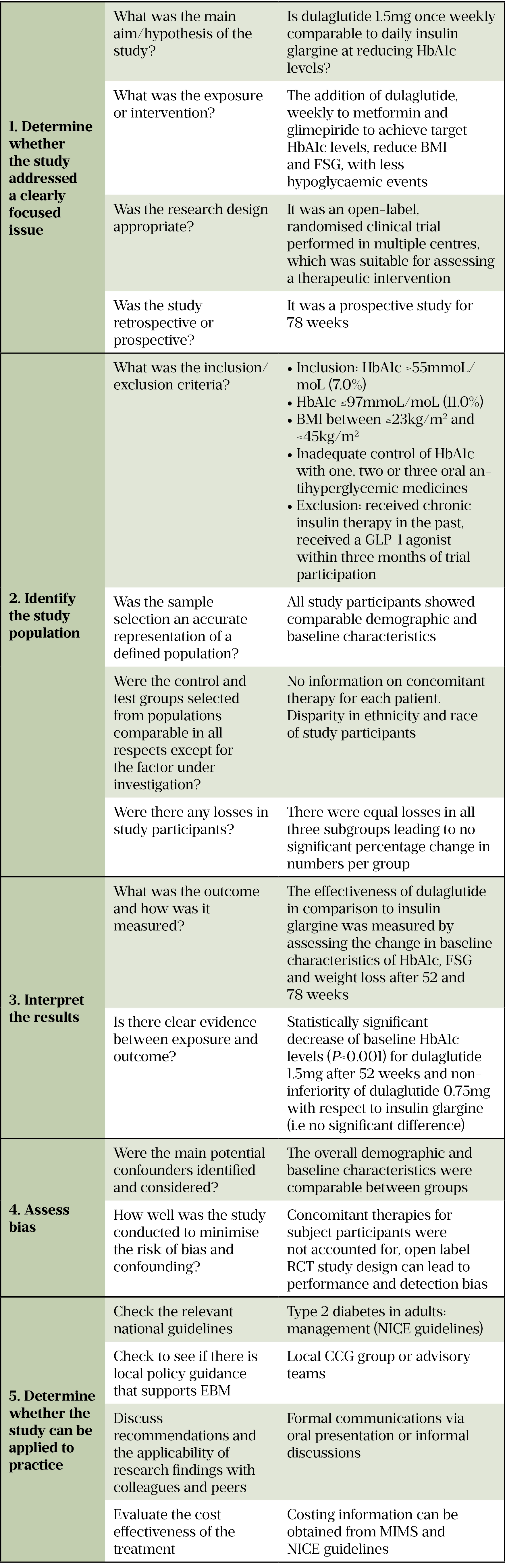
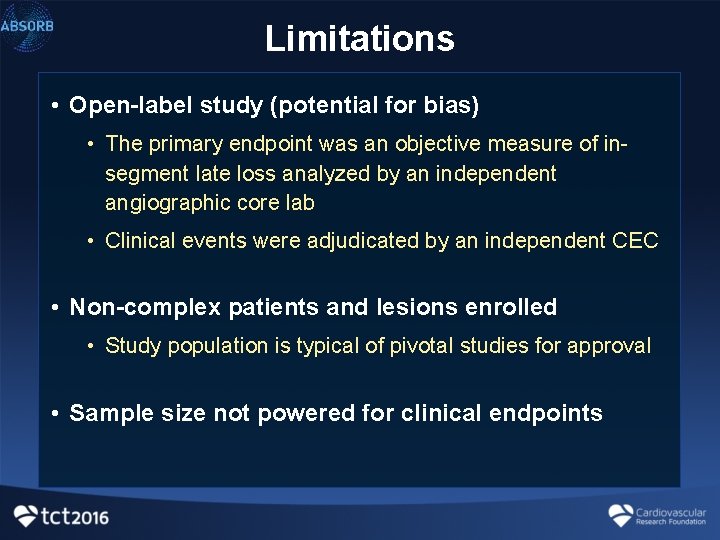
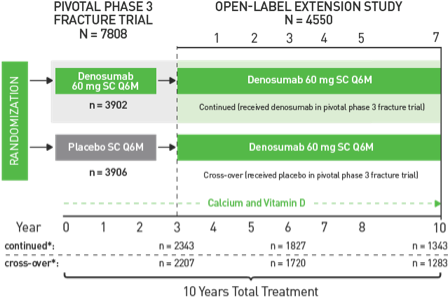
Hydroxychloroquine for COVID-19: What do the clinical trials tell us? - The Centre for Evidence-Based Medicine

Design characteristics, risk of bias, and reporting of randomised controlled trials supporting approvals of cancer drugs by European Medicines Agency, 2014-16: cross sectional analysis | The BMJ

Statistical controversies in clinical research: limitations of open-label studies assessing antiangiogenic therapies with regard to evaluation of vascular adverse drug events—a meta-analysis - Annals of Oncology
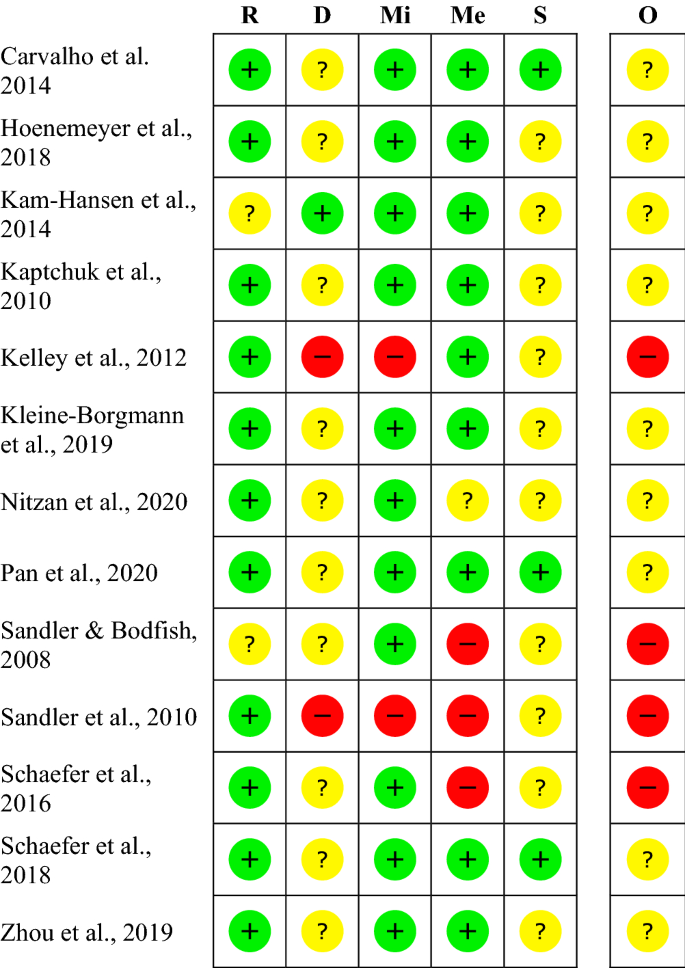
Effects of open-label placebos in clinical trials: a systematic review and meta-analysis | Scientific Reports

Bias was reduced in an open-label trial through the removal of subjective elements from the outcome definition - Journal of Clinical Epidemiology

Design characteristics, risk of bias, and reporting of randomised controlled trials supporting approvals of cancer drugs by European Medicines Agency, 2014-16: cross sectional analysis | The BMJ

Design characteristics, risk of bias, and reporting of randomised controlled trials supporting approvals of cancer drugs by European Medicines Agency, 2014-16: cross sectional analysis | The BMJ

The SANAD II study of the effectiveness and cost-effectiveness of valproate versus levetiracetam for newly diagnosed generalised and unclassifiable epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomised controlled trial - The Lancet
![PDF] Bias was reduced in an open-label trial through the removal of subjective elements from the outcome definition. | Semantic Scholar PDF] Bias was reduced in an open-label trial through the removal of subjective elements from the outcome definition. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/814ad6ccbfa9defca4d2b00c4672f9070cf6b8da/16-Figure1-1.png)
PDF] Bias was reduced in an open-label trial through the removal of subjective elements from the outcome definition. | Semantic Scholar
Effects of open-label placebos on test performance and psychological well-being in healthy medical students: a randomized controlled trial | Scientific Reports
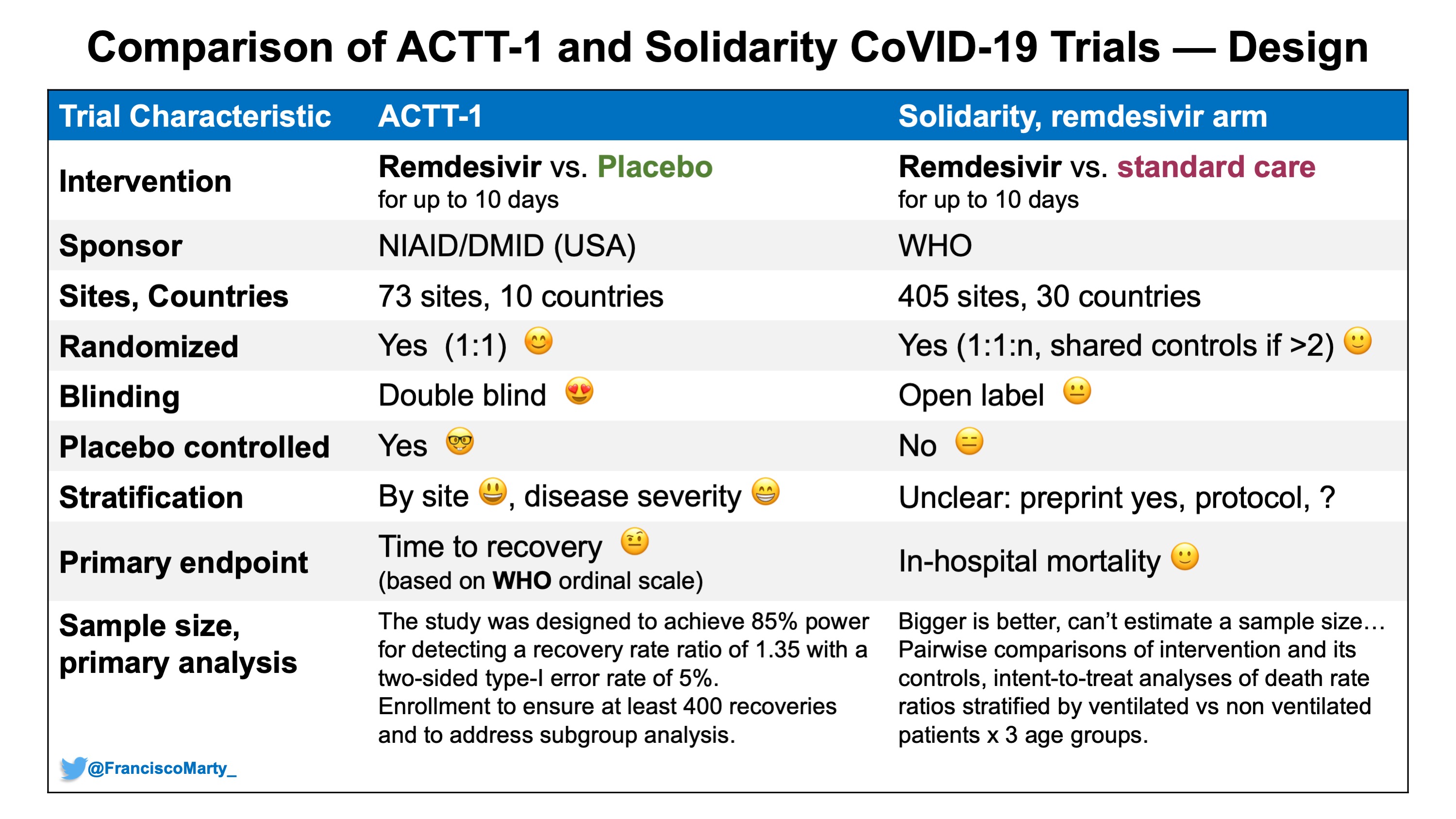
Francisco Marty, MD on Twitter: "Here is a first table comparing some basic design characteristics between #ACTT1 and #SolidarityTrial - major difference the open-label design in Solidarity vs. concealed allocation and double-blind
![PDF] The Clinical Viewpoint: Definitions, Limitations of RECIST, Practical Considerations of Measurement | Semantic Scholar PDF] The Clinical Viewpoint: Definitions, Limitations of RECIST, Practical Considerations of Measurement | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/0d341492d445073e64d1ccb6985e4ad763a3d1f7/2-Table1-1.png)
PDF] The Clinical Viewpoint: Definitions, Limitations of RECIST, Practical Considerations of Measurement | Semantic Scholar

PDF) Reducing bias in open-label trials where blinded outcome assessment is not feasible: Strategies from two randomised trials